type of intervention
Interventional - Medical Device

recruitment status
Recruiting

region
NSW, QLD, TAS, VIC, WA
type of ms
All forms of MS, People with any form of MS, Primary progressive MS, Relapsing remitting MS, Secondary progressive MS
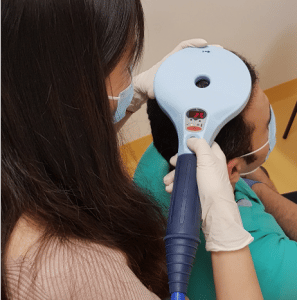
We are looking for 108 people with MS in Australia for the TAURUS.2 trial. We will measure the effects of magnetic brain stimulation (MBS for short) on multiple sclerosis (MS). MBS can non-invasively and painlessly activate nerve cells in the human brain and can be used to change nerve cell activity. MBS is currently used to treat depression but has only been used for MS in the research setting.
What’s involved
A total of 23 visits over the study duration of 4 to 5 months.
1. A full medical history, physical examination, and extended disability status score (EDSS). This will take about 30 minutes.
2. You will be randomly assigned to a treatment group, like flipping a coin. You will have a 67% chance of being in the MBS group and a 33% chance of being in the placebo group. The placebo is a fake MBS, it looks and feels like the real thing, with no brain stimulation. You will not know which group you are in until the study is completed.
3. Daily MBS or placebo sessions (Mon-Fri) for 4 to 5 weeks, at a time convenient to you. A total of 20 sessions, each one takes about 15 minutes. We will monitor side effects, new symptoms and changes in your medications. You can continue taking your current medications whilst in this study but must tell us about any changes, any GP, hospital or emergency visits, or any COVID-19 symptoms or tests.
4. Magnetic Resonance Imaging (MRI) before and after your 20 MBS or placebo sessions. Each MRI takes about an hour.
5. A series of questionnaires before and after your 20 MBS or placebo sessions. You will repeat these online after 3 months. Questionnaires take about 30 minutes to complete and include.
a. hospital anxiety and depression score (HADS), 14 questions
b. quality of life assessment (AQoL-8D), 35 questions
c. fatigue severity scale (FSS), 9 questions
d. sleep quality assessment (PQSI & NRS), 7 questions
6. MS Functional tests: Timed 25-foot walking test (T25-FW), Symbol Digit Modality Test (SDMT) and 9 Hole Peg Test (9HPT) before, during and after your MBS or placebo sessions. You will complete 3 practice sessions before starting the trial. These tests will assess arm and leg mobility as well as brain function and processing speed. The three tests take about 30 minutes to complete. You can use a walking stick or aid during the walking test.
The aims of the study are:
1. To determine whether magnetic brain stimulation improves the MS functional composite score for people living with MS.
2. To determine if magnetic brain stimulation.
– is safe & tolerable in people with MS.
– improves the quality of life in people with MS.
– reduces anxiety and depression in people with MS.
– reduces fatigue in people with MS.
– improves sleep quality in people with MS
3. To determine if magnetic brain stimulation can effectively promote remyelination in people with MS and if remyelination correlates with improvements in functional or patient-reported outcomes.
(i) Age 18-65 (inclusive)
(ii) Diagnosed with MS by an MS neurologist (McDonald criteria 2018)
(iii) Stable and relapse-free for 6 months (either on or off MS treatment)
(iv) Extended Disability Status Scale (EDSS) between 1.5 and 6 (inclusive)
(v) Willing to travel to the site every weekday for 4 consecutive weeks
(vi) Capacity to provide informed consent
(vii) Access to the internet
(i) Have metal in your body that prevents you from having an MRI scan.
(ii) Are pregnant or intend to become pregnant.
(iii) Have a history of seizures, epilepsy, stroke, brain surgery, bipolar, mania, claustrophobia, or (serious head trauma*, substance abuse*, migraines* at PI discretion)
(iv) Currently taking tricyclic antidepressants, neuroleptics, antipsychotics or antiseizure medication for the treatment of any indications listed iii.
(v) Have an EDSS <1.5 and >6.
(vi) Previously received any form of MBS (to maintain blinding).
(vii) English illiterate (where access to a suitable translator is not possible).
(viii) Currently involved in another interventional clinical trial.
*Note: These participants may be included on Principal Investigators discretionary basis.
Already commenced.
Hobart
Kate Probert Clinical Trials Study Coordinator, Multiple Sclerosis (MS)
Email: katherine.probert@utas.edu.au
Ph: (03) 6226 7746
Dr Vincent Chigozie Ezegbe, Clinical Research Fellow, Multiple Sclerosis (MS) Flagship
Email: vincent.ezegbe@utas.edu.au
Ph: (03) 6226 4236
Mr Amin Zarghami, Graduate Research Student
Email: Amin.Zarghami@utas.edu.au
Ph: (03) 6226 4656
Melbourne
Gerard Loquet
Alfred Health
55 Commercial Rd, Melbourne VIC 3004
Email: G.Loquet@alfred.org.au
May Ho
Alfred Health
55 Commercial Rd, Melbourne VIC 3004
Email: ma.ho@alfred.org.au
Newcastle
Kira Groen
John Hunter Hospital, Hunter New England Health
Lookout Rd, New Lambton Heights NSW 2305
Email: kira.groen@health.nsw.gov.au
Launceston
Monika O’Connor
The Launceston General Hospital
274-280 Charles St, Launceston TAS 7250
Email: monika.oconnor@ths.tas.gov.au
Perth
Petrina Keating
Perron Institute for Neurological and Translational Science
RR Block, QE II Medical Centre,
8 Verdun St, Nedlands, WA 6009
Email: petrina.keating@perron.uwa.edu.au
Brisbane
Tamara Powell
The Mater Research
Level 3, Aubigny Place, Raymond Terrace
South Brisbane, Qld 4101
Email: tamara.powell@mater.uq.edu.au
Full details of the trial can be found on the ANZCTR clinical trials database or clinicaltrials.gov: ACTRN12622000064707
NSW, VIC, WA, QLD, TAS (Coordinating site)
Yes. The University of Tasmania, Human Research Ethics Committee (ref: H0026359) and the Hunter New England, Human Research Ethics Committee (ref: 2022_ETH01144) (national mutual acceptance) have approved this study.
10/10/2023

